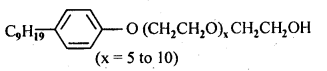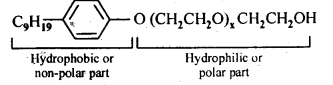Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 16 Chemistry in Everyday Life:
| Section Name | Topic Name |
| 16 | Chemistry in Everyday Life |
| 16.1 | Drugs and their Classification |
| 16.2 | Drug-Target Interaction |
| 16.3 | Therapeutic Action of Different Classes of Drugs |
| 16.4 | Chemicals in Food |
| 16.5 | Cleansing Agents |
NCERT INTEXT SOLUTIONS
16.1 Sleeping pills are recommended by doctors to the patients suffering from sleeplessness but it is not advisable to take its doses without consultation with the doctor. Why?
Ans. Most of drugs taken in doses higher than recommended may produce harmful effects and act as poison and cause even death. Therefore, a doctor must always be consulted before taking the drug.
16.2 With refrence to which classification has the statement “ranitidine is an antacid”, been given?
Ans. This statement refers to the classification of drugs according to pharmacological effect because any drug which will be used to neutralise the excess acid present in the stomach will be called an antacid.
16.3 Why do we require artificial sweetening agents?
Ans. To reduce calorie intake and to protect teeth from decaying, we need artificial sweeteners.
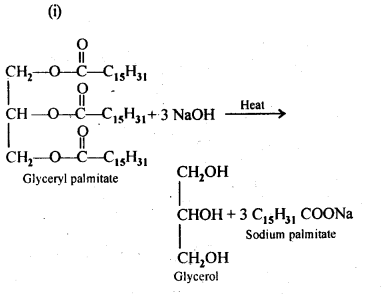
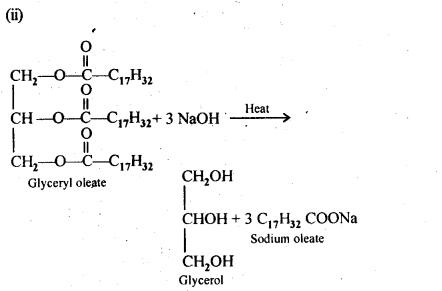
16.4 Write the chemical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structures of these compounds are given below:
(i)(C15H31COO)3C3H5-Glyceryl palmitate
(ii)(C17H32COO)3C3H5-Glyceryl oleate
Ans.
16.5 Following type of non-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic part in the molecule. Identify the functional group (s) present in the molecule.
Ans.
Functional groups present in the detergent molecule are:
(i)ether
(ii)1°alcoholic group
NCERT EXRECISES SOLUTIONS
16.1 Why do we need to classify drugs in different ways?
Ans.Drugs are classified in following different ways:
(a)Based on pharmacological effect.
(b)Based on action on a particular biochemical process.
(c)Based on chemical structure.
(d)Based on molecular targets.
Each classification has its own usefulness.
(а)Classification based on pharmacological effect is useful for doctors because it provides them the whole range of drugs available for the treatment of a particular disease.
(b)Classification based on action on a particular biochemical proc*ess is useful for choosing the correct compound for designing the synthesis of a desired drug.
(c)Classification based on chemical structure helps us to design the synthesis of a number of structurally similar compounds having different substituents and then choosing the drug having least toxicity.
(d)Classification on the basis of molecular targets is useful for medical chemists so that they can design a drug which is most effective for a particular receptor site.
16.2 Explain the term, target molecules or drug targets as used in medicinal chemistry.
Ans. Drugs interact with macromolecules like proteins, carbohydrates, lipids and nucleic acids thus these macro molecules are called drug targets. These macromolecules perform various functions in the body for example, proteins perform several roles in the body. Proteins which act as biological catalysts are called enzymes, those which are involved in communication system are called receptors. Carrier proteins carry polar molecules across the cell membrane. Nucleic acids have coded genetic information in the cell whereas lipids and carbohydrates form structural part of cell membranes.
16.3 Name the macro molecules that are chosen as drug targets.
Ans. Proteins, carbohydrates, lipids and nucleic acids are chosen as drug targets.
16.4 Why should not medicines be taken without consulting doctors?
Ans. Some drugs can cause side effects when drug binds to more than one type of receptor. Therefore, doctor’s consultation is must to choose the right drug that has the maximum affinity for a particular receptor site to have desired effect. Dose of the drug taken at a time is also crucial because some drugs in higher doses act as poisons and may cause death.
16.5 Define the term chemotherapy.
Ans. It is the branch of chemistry that deals with the treatment of diseases by using chemicals as medicines.
16.6 Which forces are involved in holding the drugs to the active site of enzymes?
Ans.The following forces are involved in holding the drugs to the active site of enzymes:
(a)Hydrogen bonding
(b)Ionic bonding
(c)Dipole-dipole interactions
(d)van der Waals interactions
16.7 While antacids and antiallergic drugs interfere with the function of histamines, why do these not interfere with the function of each other?
Ans. Drugs are designed to cure some ailment in one organ of the body do not affect the other because they work on different receptors. For example, secretion of histamine causes allergy. It also causes acidity due to release of hydrochloric acid in the stomach. Since antiallergic and antacids drugs work on different receptors, therefore, antihistamines remove allergy while antacids remove acidity.
16.8 Low level of noradrenaline is the cause of depression. What type of drugs are needed to cure this problem? Name two drugs.
Ans.In case of low level of neurotransmitter, . noradrenaline, tranquilizer (antidepressant) drugs are required because low levels of noradrenaline leads to depression. These drugs inhibit the enzymes which catalyse the degradation of noradrenaline. If the enzyme is inhibited, noradrenaline is slowly metabolized and can activate its receptor for longer periods of time thereby reducing depression. Two important drugs are iproniazid and phenylzine.
16.9 What is meant by the term broad spectrum antibiotics? Explain.
Ans. Broad spectrum antibiotics are effective against several different types or wide range of harmful bacteria. For example, tetracycline, chloramphenicol and of loxacin. Chloramphenicol can be used in case of typhoid, dysentry, acute fever, urinary infections, meningitis and pneumonia.
16.10 How do antiseptics differ from disinfectants? Give one example of each.
Ans. Antiseptics are chemical substances which prevent the growth of micro-organisms and may even kill them but they are not harmful for human or animal tissues. For example, dettol and savlon. They are generally applied on wounds, cuts, ulcers and diseased skin surfaces. Furacin and soframycin are well known antiseptic creams.
Disinfectants are chemical substances which kill microorganisms but are not safe to be applied to the living tissues. These are generally used to kill microorganisms present in the drains toilets, floors, etc. Some common examples of disinfectants are phenol ( 1% solution) and chlorine (0.2 to 0.4 ppm).
16.11 Why are cimetidine and ranitidine better antacids than sodium hydrogencarbonate or magnesium or aluminium hydroxide?
Ans. If excess of NaHCO3 or Mg(OH)2 or Al(OH)3 is used, it makes the stomach alkaline and thus triggers the release of even more HCl which may cause ulcer in the stomach. In contrast, cimetidine and ranitidine prevent the interaction of histamine with the receptor cells in the stomach wall and thus release of HCl will be less as histamine stimulates the secretion of acid.
16.12 Name a substance which can be used as an antiseptic as well as disinfectant.
Ans. 0.2% solution of phenol acts as antiseptic while 1% solution acts as a disinfectant.
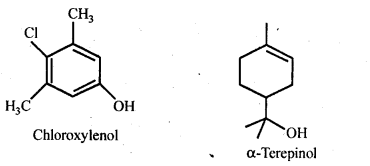
16.13 What are the main constituents of dettol?
Ans. Chloroxylenol .and α-terpineol in a suitable solvent.
16.14 What is tincture of iodine? What is its use?
Ans. 2-3% solution of iodine in alcohol and water is called tincture of iodine. It is a powerful antiseptic. It is applied on wounds.
16.15 What are food preservatives?
Ans. Chemical substances which are used to protect food against bacteria, yeasts and moulds are called preservatives. For example, sodium benzoate and sodium metabisulphite.
16.16 Why is the use of aspartame limited to cold foods and drinks?
Ans. This is because it decomposes at baking or cooking temperatures and hence can be used only in cold foods and drinks as an artificial sweetener
16.17 What are artificial sweetening agents? Give two examples.
Ans. Artificial sweeteners are chemical substances which are sweet in taste but do not add any calories to our body. They are excreted as such through urine. For example, saccharin, aspartame, alitame etc.
16.18 Name the sweetening agent used in the preparation of sweets for a diabetic patient.
Ans. Saccharine, aspartame or alitame may be used in the preparation of sweets for a diabetic patient.
16.19 What problem arises in using alitame as artificial sweetener?
Ans. Alitame is a high potency artificial sweetener.Therefore, it is difficult to control the sweetness of the food to which it is added.
16.20 How are synthetic detergents better than soaps?
Ans. They can be used in hard water as well as in acidic solution. The reason being that sulphonic acids and their calcium and magnesium salts are soluble in water thus they do not form curdy white precipitate with hard water but the fatty acids and their calcium and magnesium salts of soaps are insoluble. Detergents also works in slightly acidic solution due to formation of soluble alkyl hydrogen sulphates. Soaps react with acidic solution to form insoluble fatty acids.
16.21 Explain the following terms with suitable examples:
(i) cationic detergents (ii) anionic detergents and (iii) non-ionic detergents
Ans. (i) Cationic detergents: These are quaternary ammonium salts, chlorides, acetates, bromides etc containing one or more long chain alkyl groups. For example, cetyltrimethyl ammonium chloride.
(ii) Anionic detergents are called so because a large part of their molecules are anions. ‘These are of two types:
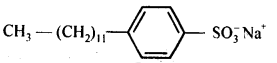
(a)Sodium alkyl sulphates: For example, sodium lauryl sulphate, C11H23CH2OSO3 Na+.
(b)Sodium alkylbenzenesulphonates.Vor example, sodium 4-(l-dodecyl) benzenesu Iphphonate (SDS).
(iii)Neutral or non-ionic detergents: These are esters of high molecular mass alcohols with fatty acids. These can also be obtained by treatment of long chain alcohols by with excess of ethylene oxide in presence of a base. For example, polyethylene glycol stearate,CH3(CH2)16COO (CH2CH2O)11 CH2CH2OH Polyethylene glycol stearate.
16.22 What are biodegradable and non-biodegradable detergents? Give one example of each.
Ans. Detergents having straight chain hydrocarbons are easily degraded (or decomposed) by microorganisms and hence are called biodegradable detergents while detergents containing branched hydrocarbon chains are not easily degraded by the microorganisms find hence are called non-biodegradable detergents. Consequently, non-biodegradable detergents accumulate in rivers and water ways thereby causing severe water pollution. Examples of biodegradable detergents are sodium lauryl sulphate, sodium 4-(-l-dodecyl) benzenesulphonate and sodium 4-(2-dodecyl) benzenesulphonate.
Examples of non-biodegradable detergents is sodium 4-(1, 3,5,7 – tetramethyloctyl) benzene sulphonate.
16.23 Why do soaps not work in hard water?
Ans. Hard water contains calcium and magnesium salts. Therefore, in hard water soaps get precipitated as calcium and magnesium soaps which being insoluble stick to the clothes as gummy mass.
16.24 Can you use soaps and synthetic detergents to check the hardness of water?
Ans. Soaps get precipitated as insoluble calcium and magnesium soaps in hard water but detergents do not. Therefore, soaps but not synthetic detergents can be used to check the hardness of water.
16.25 Explain the cleansing action of soaps.
Ans. Cleansing action of soaps : Soaps contain two parts, a large hydrocarbon which is a hydrophobic (water repelling) and a negative charged head, which is hydrophillic (water attracting). In solution water molecules being polar in nature, surround the ions & not the organic part of the molecule. When a soap is dissolved in water the molecules gather together as clusters, called micelles. The tails stick inwards & the head outwards. The hydrocarbon tail attaches itself to oily dirt. When water is agitated, the oily dirt tends to lift off from the dirty surface & dissociates into fragments. The solution now contains small globules of oil surrounded detergent molecules. The negatively charged heads present in water prevent the small globules from coming together and form aggregates. Thus the oily dirt is removed from the object.
16.26 If water contains dissolved calcium hydrogencarbonate, out of soaps and synthetic detergents, which one will you use for cleaning clothes?
Ans. Calcium hydrogencarbonate makes water hard. Therefore, soap cannot be used because it gets precipitated in hard water. On the other hand, a synthetic detergent does not precipitate in hard water because its calcium salt is also soluble in water. Therefore, synthetic detergents can be used for cleaning clothes in hard water.
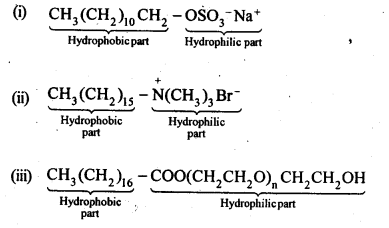
16.27 Label the hydrophilic and hydrophobic parts in the following compounds.
(i)CH3(CH2)10CH2OSO3 –Na+
(ii)CH3(CH2)15 -N+(CH3)3Br–
(iii)CH3(CH2)16C00(CH2CH2O)11CH2CH2OH
Ans.